
Image Source
Sun Pharma News:
On May 30, Sun Pharmaceutical shares saw a decline in value following rumors that one of the pharmaceutical company’s manufacturing facilities had received four observations from the US Food & Drug Administration (US FDA).
As to CNBC-TV18, Sun Pharma’s Dahej facility was inspected by the US FDA from May 10 to May 17. Subsequently, the FDA issued four observations along with the Form 483.
Sun Pharma shares were trading at Rs 1,459.4 apiece at 1:30 pm on the NSE, 1.3 percent below the closing price of the previous session.
Reports state that the USFDA noted a number of things regarding Sun Pharma. First, the company did not take the necessary safety measures to guard against API contamination. An additional remark brought attention to the absence of protocols for regular maintenance.
Sun Pharma latest update
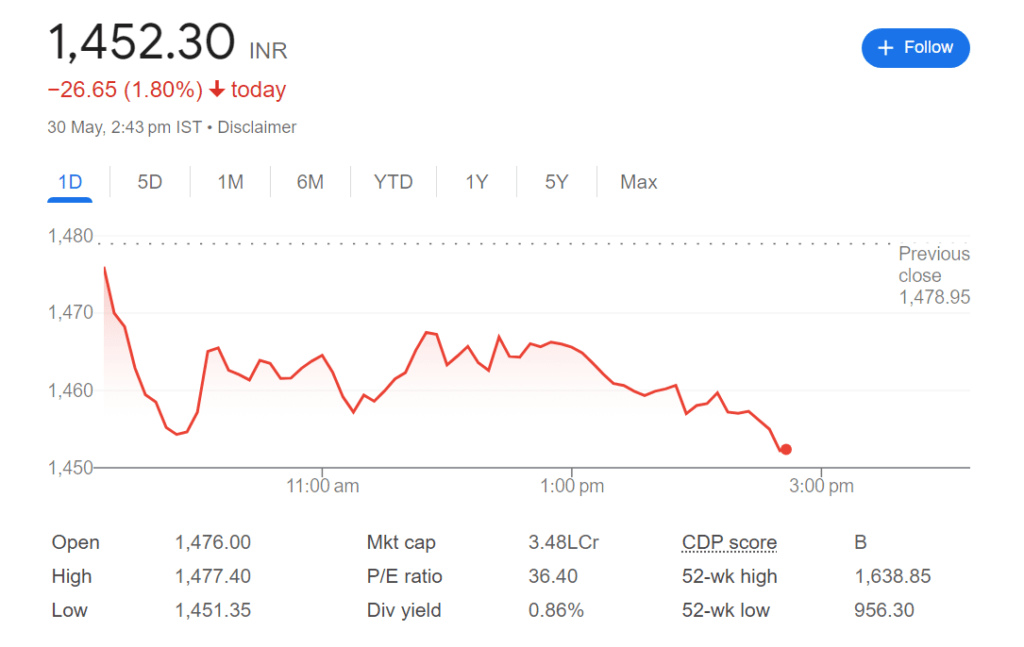
Image Source
Furthermore, Sun Pharma neglected to record their procedures for material sampling. Additionally, the business was told to determine impurity reference standards prior to analytical standards. A reference-standard substance needs to be a highly pure and well-characterized molecule, according to the USFDA.
Dahej is an important division for Sun Pharma since it makes a substantial contribution to the company’s exports to the US, which is one of its main markets. The precise revenue contributions of individual units are not disclosed by Sun Pharma.
In the last year, Sun Pharma’s stock has increased by around 52%, while the Nifty 50 index has increased by 22%.
Further Read: What is bitcoin mining?




